2025-07-07
[public] 73.0 views, 11.0 likes, dislikes audio only
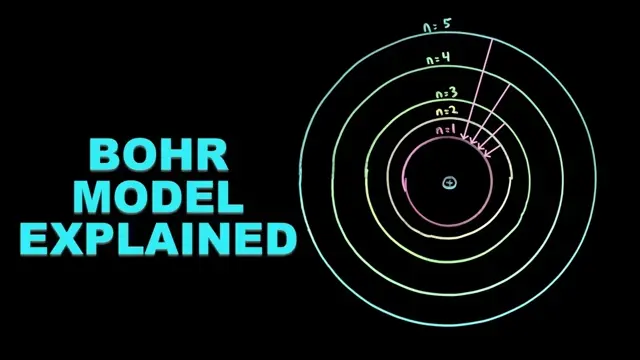
4KUnlock the secrets of the Bohr model of the atom! In this video, you'll learn how electrons occupy energy levels, how they jump between orbits, and how those transitions produce the colorful hydrogen emission spectrum.
What is an atom? Watch the whole playlist: https://www.youtube.com/playlist?list=PLvUW25kBaMMszGN5XY0QXNP_t-9Z-B8C2
Perfect for general chemistry students, this clear explanation covers:
0:00 The Problem with Rutherford's Planetary Model
1:08 Bohr's Questions
2:06 Planck & Einstein's Quantized Energy
3:50 Bohr's Atomic Model
4:43 Atomic Emission Spectra Explained
5:55 Lyman, Balmer, & Paschen Series
6:22 Rydberg Constant, Equations, & Groundbreaking Findings
6:50 Limitations of Bohr Model
Related practice problems 👉 /youtube/video/3TUGRJgqG3k
Playlists to ACE Chemistry:
https://www.youtube.com/playlist?list=PLvUW25kBaMMssGHFrR4Ee7_-8cFBvBkgN
https://www.youtube.com/playlist?list=PLvUW25kBaMMs9m0GL4dz8jSs0K4PWfqCp
https://www.youtube.com/playlist?list=PLvUW25kBaMMvs5nsG1VUQA0wsP9LGEQha
https://www.youtube.com/playlist?list=PLvUW25kBaMMuKRkW9gDV7vlAk4Y9WRU7c
https://www.youtube.com/playlist?list=PLvUW25kBaMMuHwUffHLOozSFQ0Cz_iTnC
https://www.youtube.com/playlist?list=PLvUW25kBaMMv7_3jTEAe3vVZqPhsjf5Oy
https://www.youtube.com/playlist?list=PLvUW25kBaMMtc45U4K2b0Dm88L9D7d0EE
PATREON: https://www.patreon.com/c/ChemistryinaNutshell
Citations:
OpenStax Chemistry 2e. Flowers, P., Theopold, K., Langley, R., & Robinson, W.R. (2019, Feb 14). Chemistry 2e. OpenStax. https://openstax.org/details/books/chemistry-2e
OpenStax Chemistry 2e, Figure 6.13 Compare the two types of emission spectra: continuous spectrum of white light (top) and the line
spectra of the light from excited sodium, hydrogen, calcium, and mercury atoms. Continuous spectrum and emission spectrum of calcium were used to show continuous and discrete spectra of a rainbow.
OpenStax Chemistry 2e, Figure 6.3 Portions of the electromagnetic spectrum are shown in order of decreasing frequency and increasing wavelength. (credit “Cosmic ray": modification of work by NASA; credit “PET scan": modification of work by the National Institute of Health; credit “X-ray": modification of work by Dr. Jochen Lengerke; credit “Dental curing": modification of work by the Department of the Navy; credit “Night vision": modification of work by the Department of the Army; credit “Remote": modification of work by Emilian Robert Vicol; credit “Cell phone": modification of work by Brett Jordan; credit “Microwave oven": modification of work by Billy Mabray; credit “AM radio": modification of work by Dave Clausen)
Images:
Niels Bohr, public domain: https://en.wikipedia.org/wiki/Niels_Bohr#/media/File:Niels_Bohr.jpg
Free B Roll provided by Videezy: http://www.videezy.com
Max Planck, public domain: https://en.wikipedia.org/wiki/Max_Planck#/media/File:Max_Planck_by_Hugo_Erfurth_1938cr_-_restoration1.jpg
Albert Einstein, public domain: https://commons.wikimedia.org/wiki/File:Albert_Einstein_sticks_his_tongue_1951.jpg
Photo by Brad Switzer: https://www.pexels.com/photo/magnificent-view-of-milky-way-from-cutler-cove-utah-usa-11716072/
Fingerprint: https://pixabay.com/photos/fingerprint-sense-template-456483/
By Photograph: JonathunderMedal: Erik Lindberg (1873-1966) - Derivative of File:NobelPrize.JPG, PD-US, https://en.wikipedia.org/w/index.php?curid=58432969
/youtube/video/ccpnNyhyxwo?t=0
/youtube/video/ccpnNyhyxwo?t=68
/youtube/video/ccpnNyhyxwo?t=126
/youtube/video/ccpnNyhyxwo?t=230
/youtube/video/ccpnNyhyxwo?t=283
/youtube/video/ccpnNyhyxwo?t=355
/youtube/video/ccpnNyhyxwo?t=382
/youtube/video/ccpnNyhyxwo?t=410
/youtube/channel/UCXPqF6wEXTcFtml-mF7_J9w
/youtube/video/mh2oO1dK5OQ
https://www.patreon.com/ChemistryinaNutshell
/youtube/video/CjwxFLbXgkY