2021-06-13
[public] 116 views, 8.00 likes, dislikes audio only
Empirical and molecular formulas both describe chemical compounds, but they’re not the same! In this video, we compare the two, explain when each is used, and show how to determine a molecular formula from an empirical formula using molar mass. Includes worked examples for clarity! Practice Problems included!
Time Stamps:
0:00 Introduction
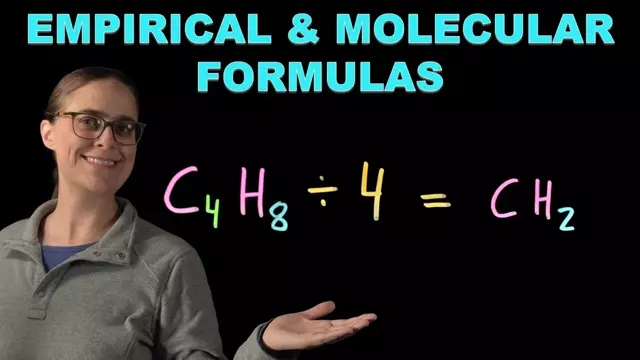
0:15 Determining empirical and molecular formulas from a Lewis structure (2-butene)
2:55 Definitions of empirical formula and molecular formula
3:41 Determining empirical and molecular formulas from a Lewis structure (C5H8)
4:40 Practice problems (3): Converting molecular formulas into empirical formulas
6:26 Different chemical compounds with the same empirical formula (formaldehyde and glucose)
#EmpiricalFormula #MolecularFormula #chemistry
/youtube/video/ZWloYVFK8w0?t=0
/youtube/video/ZWloYVFK8w0?t=15
/youtube/video/ZWloYVFK8w0?t=175
/youtube/video/ZWloYVFK8w0?t=221
/youtube/video/ZWloYVFK8w0?t=280
/youtube/video/ZWloYVFK8w0?t=386
/youtube/channel/UCXPqF6wEXTcFtml-mF7_J9w
/youtube/video/qc7xKtLq5bs
/youtube/video/9pRMcyDx2CE