2010-07-08
[public] 92.0K views, 1.41K likes, 19.0 dislikes audio only
More links in description below ↓↓↓
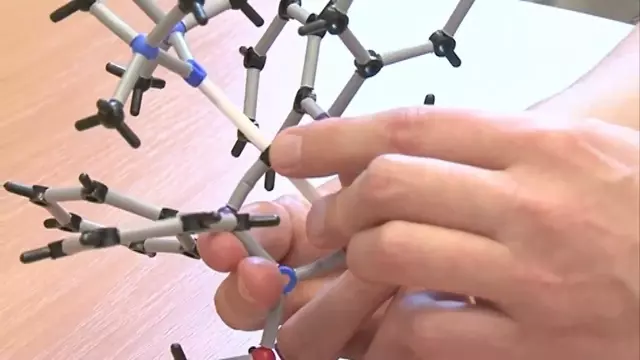
How can you create traditional methane-style carbon bonds which are planar (flat) rather than the traditional tetrahedral shape. Dr Stephen Liddle explains. His work (and our molecular video series) are supported by the EPSRC (Engineering and Physical Sciences Research Council).
Support Periodic Videos on Patreon: https://www.patreon.com/periodicvideos
A video on every element: http://bit.ly/118elements
More at http://www.periodicvideos.com/
Follow us on Facebook at http://www.facebook.com/periodicvideos
And on Twitter at http://twitter.com/periodicvideos
From the School of Chemistry at The University of Nottingham: http://bit.ly/NottChem
Periodic Videos films are by video journalist Brady Haran: http://www.bradyharanblog.com
Join Brady's mailing list for updates and extra stuff --- http://eepurl.com/YdjL9