2017-03-03
[public] 605K views, 20.4K likes, 132 dislikes audio only
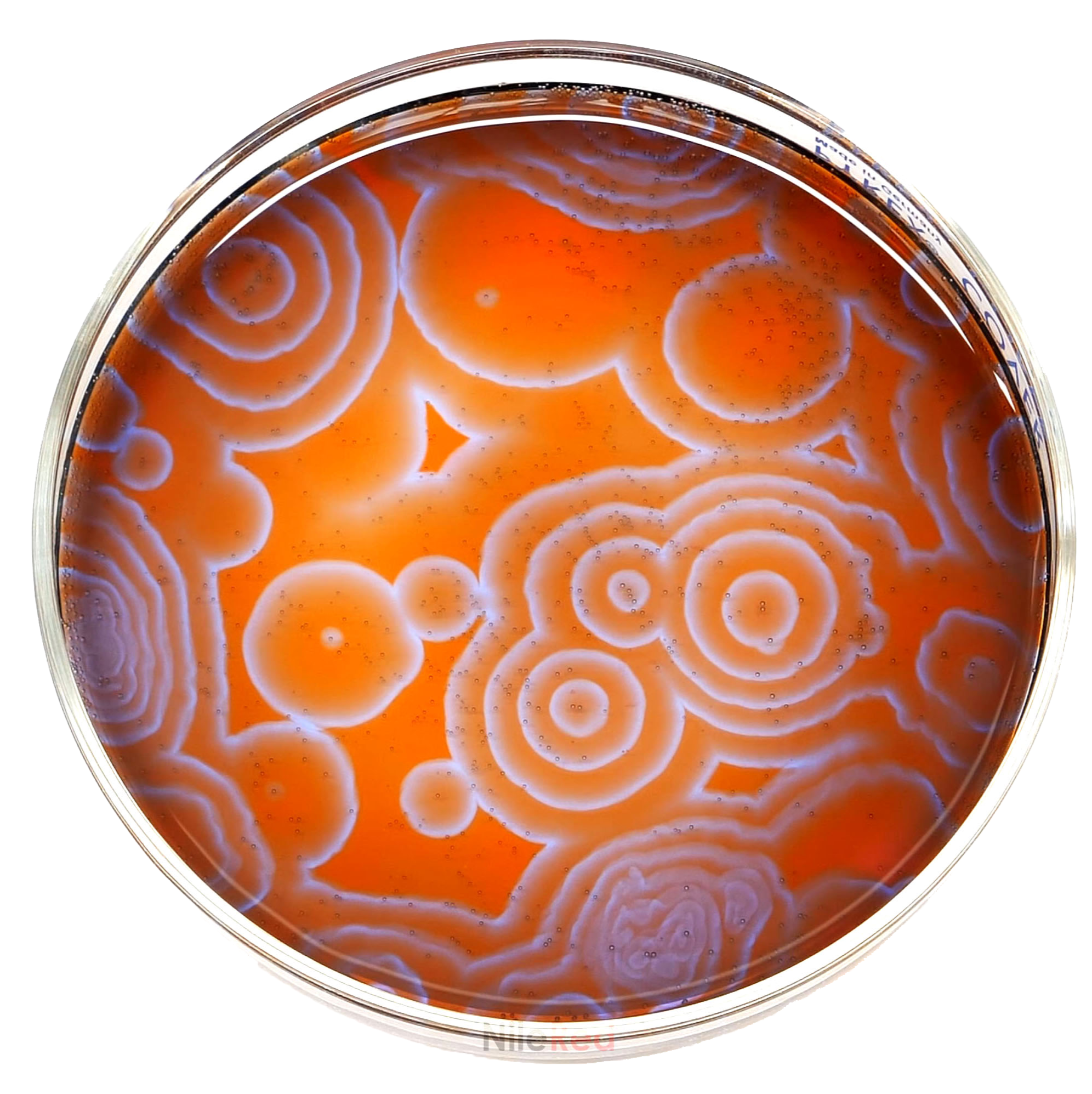
The Briggs-Rauscher oscillating reaction is one of the very few oscillating reactions that we know of.
Throughout the reaction, the concentration of iodide (I-) goes up and down, and this causes a swap between 2 processes. The the concentration is high, the process that consumes iodide is favored. When it is low, the process that produces it is favored. This causes a constant fluctuation of iodide.
As the iodide increases, it forms I2 which is yellow, then the I2 eventually combines with I- to form I3. This complexes with starch and forms a blue-black complex. The iodide producing process is then shutdown and the process that consumes it takes over. The iodide concentration drops, the I3-starch complex falls apart and the color dissipates. The I2 is sequestered by malonic acid and the solution reverts to colorless.
The [i-] continues to fall until the other process then takes over. Iodide is produced again and the cycle repeats itself.
NOTE: The yellow color of solution A was solved almost instantly by Bill Smathers. The iodate was likely contaminated with iodide. In acid, the lead to the production of I2, which gave the solution a yellow color. Upon addition of B, the I2 was sequestered by malonic acid!
Belousov video: /youtube/video/LL3kVtc-4vY
-------------------------------------------------------------------------------
Merch - https://nilered.tv/store
-------------------------------------------------------------------------------
■ NileRed is now available on Nebula! https://go.nebula.tv/nilered
(when signing up with this link, a portion of your membership directly supports the channel)
Join the community:
Patreon - https://www.patreon.com/nilered
Discord - https://discord.com/invite/3BT6UHf
NileRed Newsletter - https://nile.red/home#newsletter
You can also find me here:
Facebook - https://m.facebook.com/NileRed2
Instagram - https://m.instagram.com/nile.red
Twitter - https://mobile.twitter.com/NileRed2
Nile talks about lab safety: https://youtu.be/ftACSEJ6DZA