2025-05-28
[public] 23.0 views, 13.0 likes, dislikes audio only
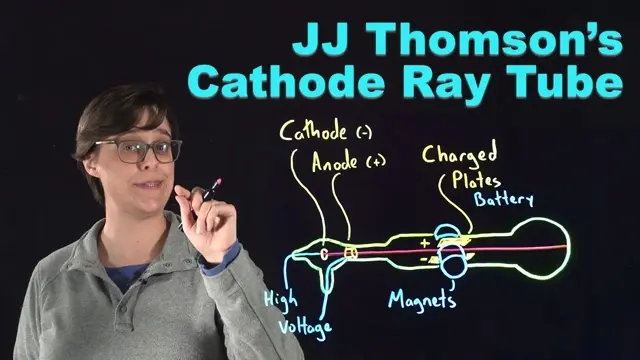
4KWith a glowing glass cathode ray tube, J.J. Thomson discovered the first subatomic particle: the electron. Learn how his experiments changed the idea of the atom forever by proving that atoms are divisible, leading to Thomson's plum pudding and Nagaoka Saturn-like model of the atom.
What is an atom? Watch the whole playlist: https://www.youtube.com/playlist?list=PLvUW25kBaMMszGN5XY0QXNP_t-9Z-B8C2
Playlists to ACE Chemistry:
https://www.youtube.com/playlist?list=PLvUW25kBaMMssGHFrR4Ee7_-8cFBvBkgN
https://www.youtube.com/playlist?list=PLvUW25kBaMMs9m0GL4dz8jSs0K4PWfqCp
https://www.youtube.com/playlist?list=PLvUW25kBaMMvs5nsG1VUQA0wsP9LGEQha
https://www.youtube.com/playlist?list=PLvUW25kBaMMuKRkW9gDV7vlAk4Y9WRU7c
https://www.youtube.com/playlist?list=PLvUW25kBaMMuHwUffHLOozSFQ0Cz_iTnC
https://www.youtube.com/playlist?list=PLvUW25kBaMMv7_3jTEAe3vVZqPhsjf5Oy
https://www.youtube.com/playlist?list=PLvUW25kBaMMtc45U4K2b0Dm88L9D7d0EE
PATREON: https://www.patreon.com/c/ChemistryinaNutshell
0:00 JJ Thomson's Cathode Ray Tube Experiment
1:10 Charge-to-Mass Ratio
1:38 Implications of Findings
2:37 TL;DR Thomson's Findings
3:11 What does this mean for the structure of the atom?
3:28 Atomic Models: Nagaoka's Saturn-Like Atom & Thomson's Plum Pudding Model
Images Used:
https://en.wikipedia.org/wiki/File:J.J_Thomson.jpg By Not Mentioned - First World War.com, Public Domain, https://commons.wikimedia.org/w/index.php?curid=2969861
/youtube/video/Ks1SqkJS7E0?t=0
/youtube/video/Ks1SqkJS7E0?t=70
/youtube/video/Ks1SqkJS7E0?t=98
/youtube/video/Ks1SqkJS7E0?t=157
/youtube/video/Ks1SqkJS7E0?t=191
/youtube/video/Ks1SqkJS7E0?t=208
/youtube/channel/UCXPqF6wEXTcFtml-mF7_J9w
/youtube/video/ptkUlZgBW6Y
https://www.patreon.com/ChemistryinaNutshell