2023-09-10
[public] 206K views, 10.4K likes, dislikes audio only
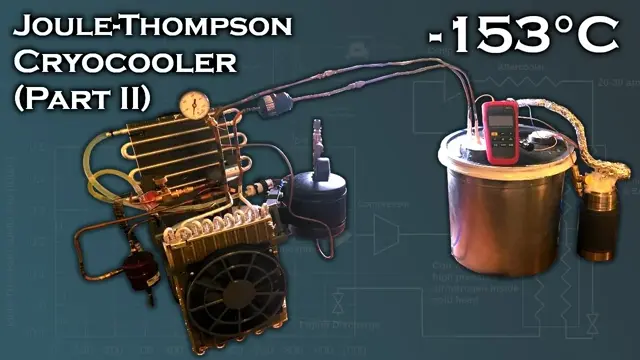
In this video, I'll build on my progress from the last Joule-Thomson
cryocooler video and reach a temperature of -153C on a single stage
using parts sourced from window A/C units and common/cheap substances as
refrigerants (a mixture of Propane, Ethylene, and Methane). This
temperature is low enough to condense any gas except for Hydrogen,
Helium, or Neon, at the following pressures:
Nitrogen - 25.3 barA (352 PsiG)
Air - 20.2 barA (278 PsiG)
Oxygen - 10.3 barA (135 PsiG)
Methane - 1.9 barA (13 PsiG)
I tested several different mixtures to achieve this temperature, but the
best performing I've found so far has been a 30/20/50 blend of Propane,
Ethylene, and Methane, respectively. The Propane comes from an ordinary
grilling tank, the Ethylene is produced by heating denatured alcohol
over a catalyst, and the Methane comes from residential natural gas. All
these sources are very cheap.
For a throttling valve to achieve the Joule-Thomson effect, I use a 1m
long 1mm ID capillary inline with a computer-controllable electronic
expansion valve (EEV) used for fine-tuning flow resistance. I avoid
using *only* an EEV due to the relatively large thermal conduction
losses experienced through the bulky metal valve body.
The system is charged with 180 PsiG (11.3 BarA). With the EEV wide open,
the high/low side pressure is ~400/65 psiG (28.6/5.5 barA) respectively.
With the EEV closed as far as possible without shutting off the flow,
the high/low side pressure is ~520/10 psiG (36.9/1.7 barA) respectively.
A temperature drop of 20-35°C across the EEV+capillary restriction is
typical.
I use a vacuum enclosure to thermally insulate the cold end of the
system, but since I can't reach a vacuum much higher than about ~2 mBar,
the vacuum isn't a very effective insulator, so I still had to pack the
enclosure and wrap the heat exchanger coil with glass wool. Research
papers on this subject claim pressures on the order of 10^-4 mBar inside
the vacuum enclosure, which I'm unable to produce.
While the temperature I've achieved is low enough to make liquid
nitrogen / oxygen / methane under pressure, the heat exchanger I
assembled to do so on the cold end was ineffective, so I didn't liquify
any gas in this video.
In the next part of this series, I'll focus on mixture optimization (by
adding varying percentages of Butane and Nitrogen or Argon to the mix),
increasing cooler power / temperature drop by use of a pre-cooler, and
construction of a cold head that incorporates a large "reservoir" for
accumulating several hundred CC of liquified gas before discharge to
atmospheric pressure.
If this project is succesful in producing decent quantities of liquid
Nitrogen, I'll turn my focus toward production of Liquid Hydrogen using
a Joule-Thomson system with LN2 pre-cooling.